What is VIZIMPRO?
VIZIMPRO® (dacomitinib) is a prescription medicine designed to treat non-small cell lung cancer (NSCLC) that has spread to other parts of the body (metastatic) and is used as a first treatment if your tumor has certain abnormal epidermal growth factor receptor (EGFR) genes. Your healthcare provider will perform a test to make sure that VIZIMPRO is right for you.
It is not known if VIZIMPRO is safe and effective in children.
How does VIZIMPRO work?
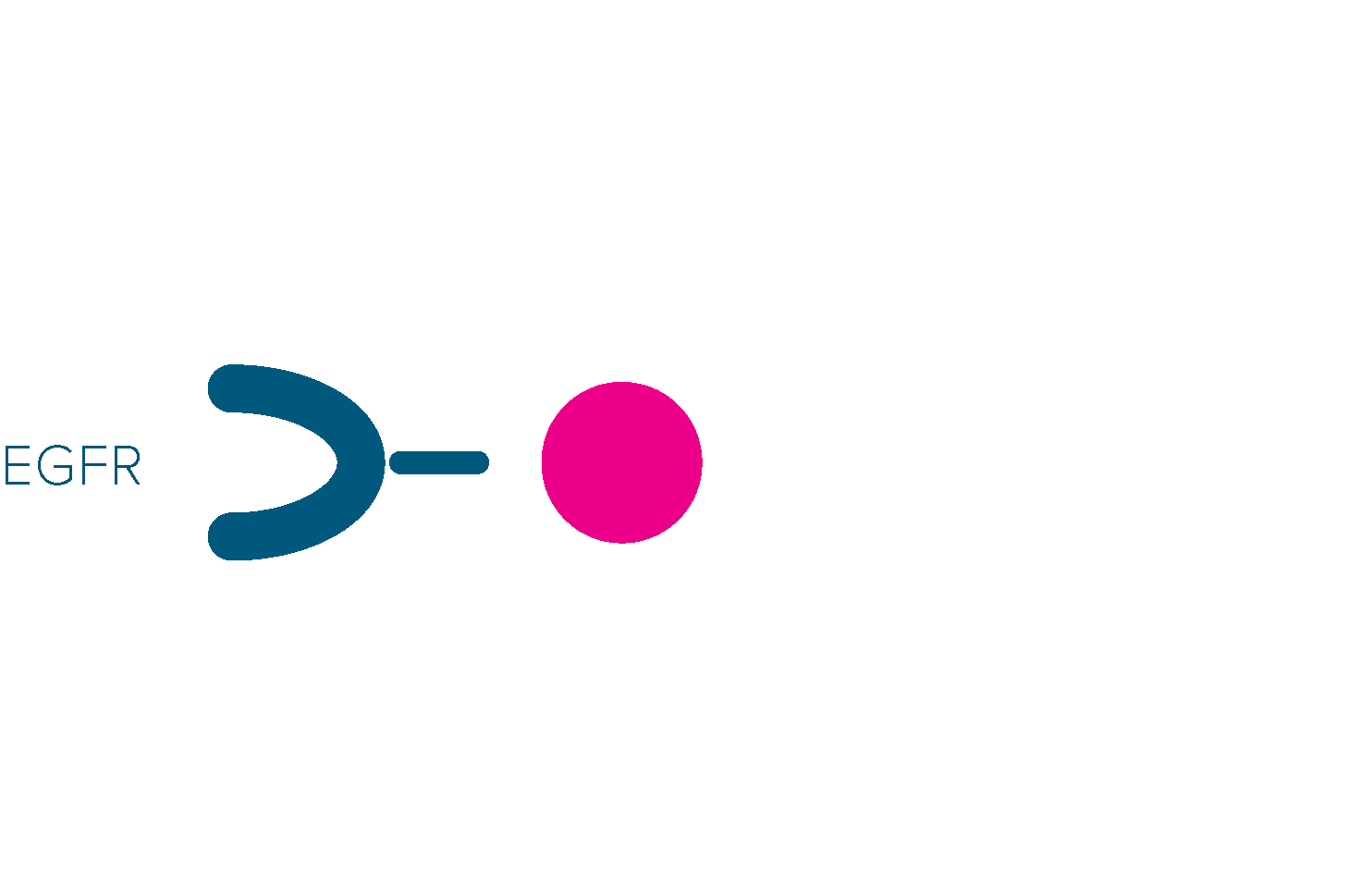
VIZIMPRO attaches to EGFR proteins, blocking signals that help cancer cells grow.
This may help shrink or slow the growth of NSCLC tumors with the EGFR exon 19 deletion mutation or the L858R substitution in exon 21 mutation. VIZIMPRO can also affect normal cells, which may contribute to some of the side effects.




How was VIZIMPRO studied?
VIZIMPRO was compared to another EGFR inhibitor (gefitinib) in a head-to-head clinical study. This type of study provides a direct comparison of the safety and effectiveness of the 2 treatments.
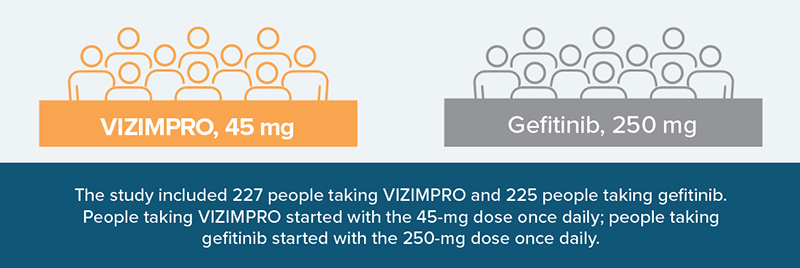
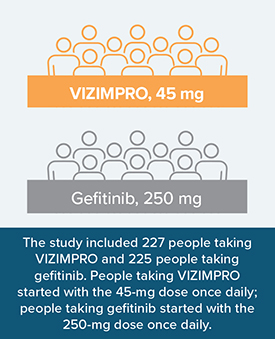
Adults in the study either had:
- No prior therapy for their EGFR-positive NSCLC that had spread to other parts of the body, or
- EGFR-positive NSCLC that came back after at least 12 months without detectable cancer following a past therapy
Effectiveness of the 2 treatments was measured in a few different ways:
- How long people lived without their cancer growing or spreading (progression-free survival)
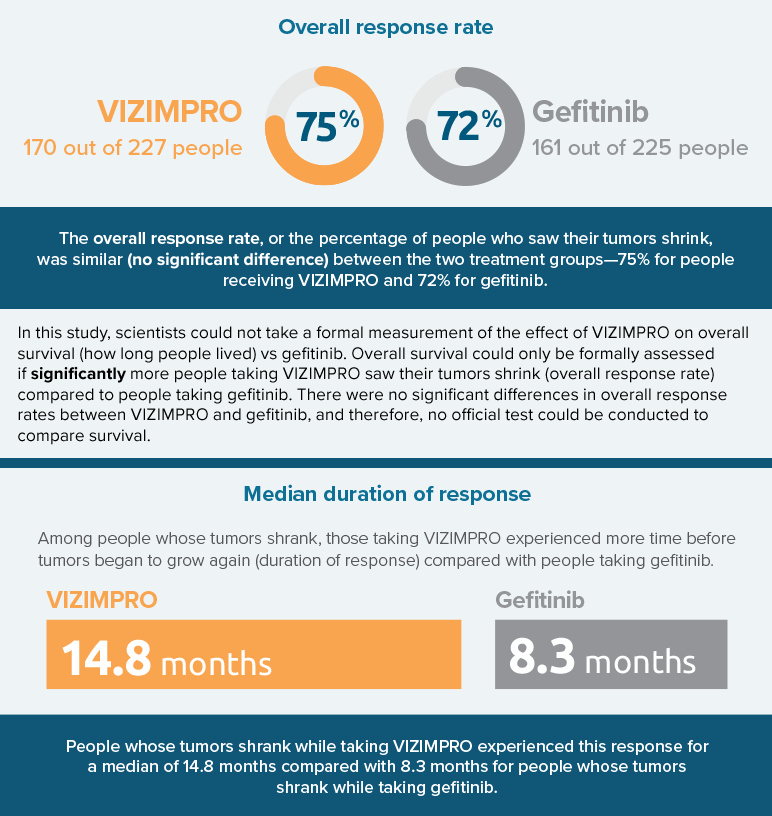
- How many people had their tumors shrink (overall response rate)
- How long responses lasted before tumors resumed growing or spreading (duration of response)
- The overall length of time people lived (overall survival)
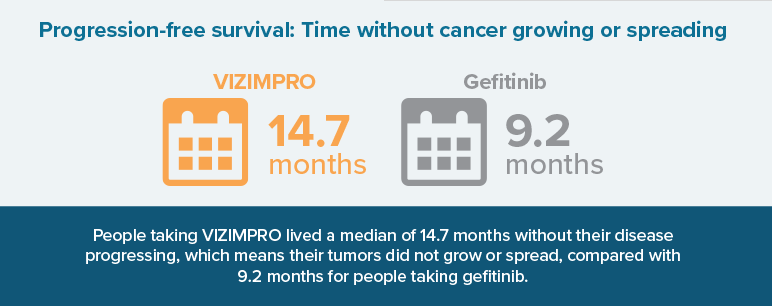
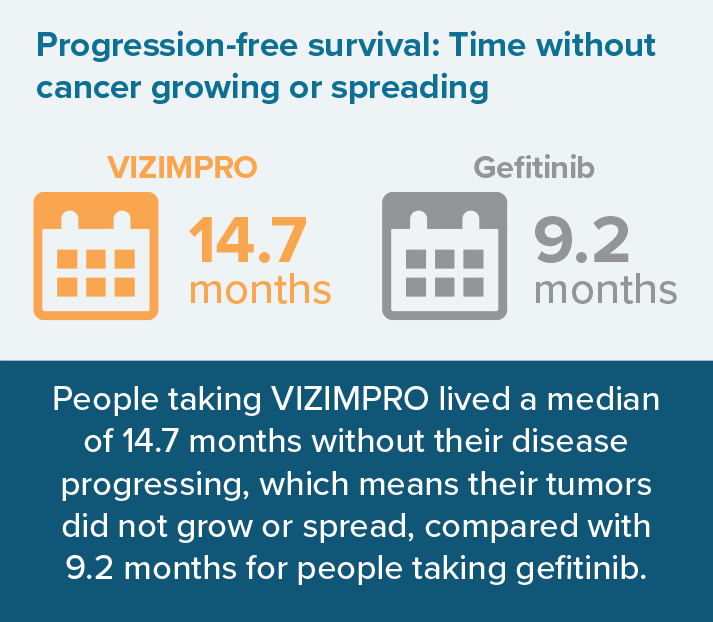
What was seen with VIZIMPRO in the clinical study?
The median is the “middle number” in a list of numbers ordered from lowest to highest. In this case, the median time to disease progression was 14.7 months, so half of people experienced disease progression before 14.7 months and half of people experienced disease progression after 14.7 months.